Welcome
Members of the Department

Bichara, Ali
514-457-6610 ext. 5990
HS-131
ali.bichara@johnabbott.qc.ca
Ali Bichara is a full time professor in Biopharmaceutical Production Technology department at John Abbott College. He is involved in teaching bioprocessing, production planning, solid and liquid dosage forms.
He has over 15 years experience in the pharmaceutical industry where he occupied different positions. He started his career as a research scientist in Biosyntech a biotech company, after that he worked for 10 years as a Senior Manager of Formulation development and was involved in the development of all globally approved products developed by Labopharm. Then he was employed by Garmen as a Director of R&D.
Prior to joining John Abbott College, he was employed by Altus Formulation as a Director of Formulation Development.
Ali holds a PhD in Polymer Chemistry from University of St-Etienne and an MBA from ESG Montreal/Paris Dauphine. He is the author and co-author for several publications and patent applications.

Cianfaglia, Luca
514-457-6610 ext. 5599
AME-132
luca.cianfaglia@johnabbott.qc.ca
Prior to joining John Abbott College’s BioPharmaceutical Production Technology department Luca Cianfaglia worked in the pharmaceutical industry for 27 years and held positions in quality control, quality assurance, validation and scientific affairs. He worked in research at the Montreal Neurological Institute-Hospital developing analytical test methods for the analysis of neuronal markers, amino acids and antiepileptic drugs in tissue cultures and biological fluids. He also worked in an analytical testing lab as an analyst where he performed extractions of performance enhancing drugs. Luca holds a Bachelor of Science degree in chemistry from McGill University and a Diploma in Education from McGill University.

Jamal, Rachid
514-457-6610 ext. 5543
AME-133
rachid.jamal@johnabbott.qc.ca
Rachid Jamal holds a BS in Chemistry as well as an M.Sc in Chemical Engineering obtained in 1989 from the University of Southern California in Los Angeles. He was a post graduate researcher at Toronto University in Chemical Engineering for two years (1992-1993). Then he started his industrial career as a supervisor in a personal and healthcare plant in Toronto where he worked for 5 years. Then he completed several Industrial Pharmaceutical courses at Seneca College in Toronto in 1999 which strengthened his pharmaceutical knowledge. After that he worked in the pharmaceutical industry for more than 16 years with various dosage forms mainly in scale-up, process development, optimization, validation and technology transfer, this was for different pharmaceutical companies located in Toronto and Montreal area. During that time he attended several intensive courses in a wide range of pharmaceutical disciplines.

Khalid, Mohamed Nabil
Chair514-457-6610 ext. 5327
AME-129
nabil.khalid@johnabbott.qc.ca
Nabil M. Khalid has been a Professor and Department Chair in the Biopharmaceutical Production Technology department at John Abbott College since 2012. Nabil holds a PhD in Pharmacy specialized in Pharmaceutical Technology from the University of Paris XI College of Pharmacy as well as an MBA from ESG University of Quebec in Montreal. After completing his PhD, he received the 1er Prix de these from the University of Paris XI and the National Academy of Pharmacy.
Since 1996, he was active in both academic and industrial sectors. He works in clinical affairs and pharmacovigilance at SmithKline Beecham in Paris. Then relocated in Montreal, he joined several Pharmaceutical companies and organizations in Canada like Labopharm as Scientist, Genome Quebec as Director Business Development for RNomic platform and BioEnvelope division Paladin labs as Director of Scientific Affairs. He Was a lecturer in charge of Sterile Products course from 2005 to 2009 at the University of Montreal, Faculty of Pharmacy and he is regularly invited lecturer at the Polytechnic School of Montreal, Biomedical Engineering Institute. He was also involved in teaching activities at Dawson College in the Biotechnology and the Nano-Biotechnology AEC as well as the ContEd Chemistry courses. He was also Associate Professor at Ajman University of Science and Technology (UAE) College of Pharmacy and Health Sciences 2010-2011 where he was teaching Pharmaceutical Technology for both undergraduate and graduate students.
Since 1994, he is involved in many pharmaceutical research and development projects mainly in the field of solid and liquid dosage forms formulation and controlled drug delivery. He is the author and co-author of many publications and patent applications.
At John Abbott College, Nabil is involved in teaching liquid manufacturing, oral solid dosage form, and bioprocessing.

Rahmouni, Miloud
514-457-6610 ext. 5599
AME-132
miloud.rahmouni@johnabbott.qc.ca
Miloud Rahmouni is a full time professor in Biopharmaceutical Production Technology department at John Abbott College. He has been teaching sterile manufacturing, quality control and production planning since 2013. Miloud holds a Ph. D. in pharmaceutical technology from university of Montreal, and has more than twenty years industrial experience in pharmaceutical formulation, process development and tech-transfer of oral solid dosage forms. His areas of expertise in pharmaceutical processing includes: compression, granulation, pelletization, film coating, microencapsulation, spray drying, freeze drying, polymeric micelles and injectable formulation. Before joining John Abbott College, Miloud worked as senior-director of production at Garmen laboratory, and associate director of R&D at Labopharm. Besides presenting lectures to undergraduate student, Miloud is actively involved in research and development of new drug delivery systems. He is author and co-author of many scientific publications and inventor on several patent applications
Courses
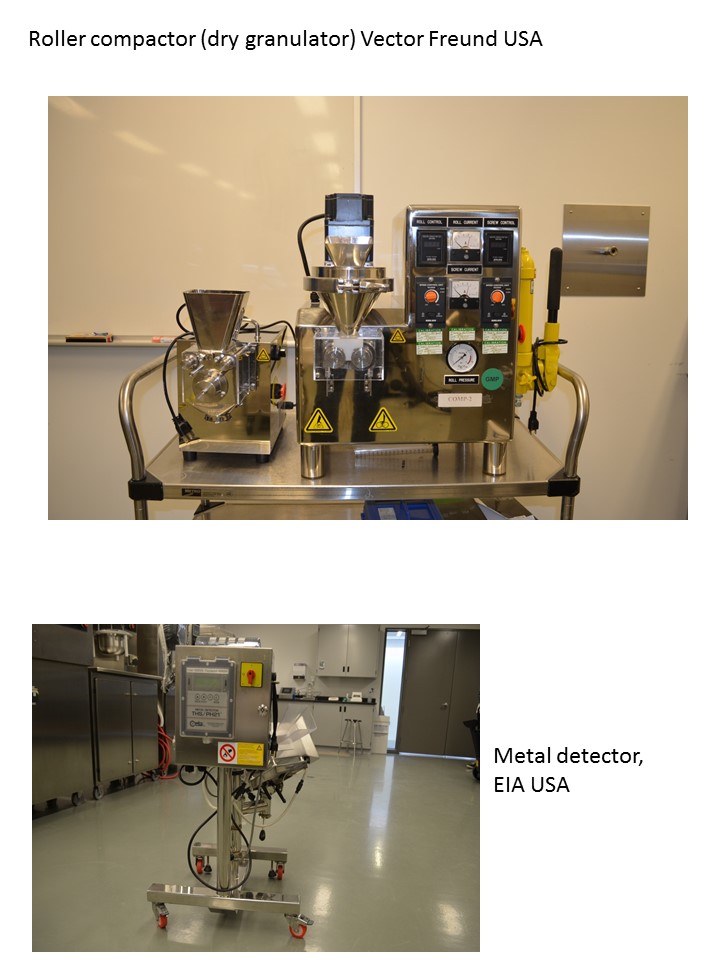
Equipment
Area of Expertise
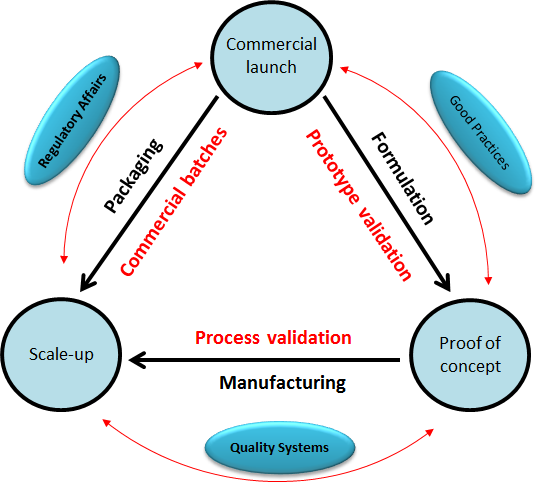
Pharmaceutical Production Technology faculty members are fully dedicated in their respective teaching and training activities. With an impressive area of expertise, we are also open to offer through the College a diversity of services to medium and small companies with the goal of stimulating our local and regional economy!
The area of expertise includes:
- Feasibility study
- Preformulation and compatibility study
- Formulation development for Oral solid Dosage Forms
- Formulation development for Liquid Dosage Forms including creams, ointments, gels and sterile
- Prototype development (generic Products)
- Physicochemical characterization of active ingredients, excipients and finished products
- Characterization and bioperformances evaluation and full Quality Control testing of raw materials and finished products (dissolution, disintegration and many others)
- Manufacturing processes optimization and improvement
- Scale-up: Lab-Pilot and Pilot-Comercial
- A la carte pharmaceutical operations (granulation, coating, lyophilisation, spheronization, tableting, capsule filling and many other processing approaches
- Analytical method development, validation and method transfer for both Quality Control and research purposes
- Quality assurance procedures design and implementation
- Coaching preparation for regulatory institutions audits (Health Canada and FDA)
- Coaching for Lab / manufacturing area modification and expansion
- Coaching for DIN and NPN submission for Health Canada and equivalent for FDA
For any further information, please contact us at:
514-457-6610 x5327
biopharma@johnabbott.qc.ca